How liquids evaporate
It is a matter of common knowledge that some water in an open vessel eventually dries up through evaporation. Liquids vary in the rate at which they evaporate at ordinary temperatures. They are said to be volatile.On the other hand, lubricating oil and mercury never seem to evaporate, however they are allowed to stand.
When discussing the process of evaporating, we used the kinetic energy to explain how molecules escape from a liquid.
If a liquid is heated, the energy which goes into it becomes mechanical energy in the molecules. More and more of the molecules gain enough kinetic energy to enable them to escape from the attraction of their neighbours and jump right out of the liquid.
A rise in temperature is, therefore, accompained by an increase in the rate of evaporation.
A rise in temperature is, therefore, accompained by an increase in the rate of evaporation.
Saturated vapour pressure (s.v.p.)
Let us suppose that some liquid is poured into a bottle which is then corked up. Owing to evaporation, the escape above the liquid begins to fill with vapour. The vapour molecules move about in all directions and exert pressure when they bounce off the walls of the bottle.They also strike the surface of the liquid and many re-enter it. Eventually a state of dynamic equilibrium is reached in which the rate at which molecules leave the liquid is equal to the rate at which others return to it.
The use of the word dynaminc to describe the equilibrium stresses the fact that the molecules are in continues motion with equal two-way traffic at the surface of the liquid.
Under these conditions the space above the liquid is said to be saturated with vapour, and the pressure exerted is called the saturation vapour pressure ( s.v.p.)..
For a given temperature the saturation vapour pressure of a liquid is always the same whether there is air in the space above it or not.
Before equilibrium has been reached in the manner described the vapour is said to be unsaturated.
A saturated vapour is one which is in a state of dynamic equilibrium with its own liquid or solid.
saturated vapour pressure of a liquid: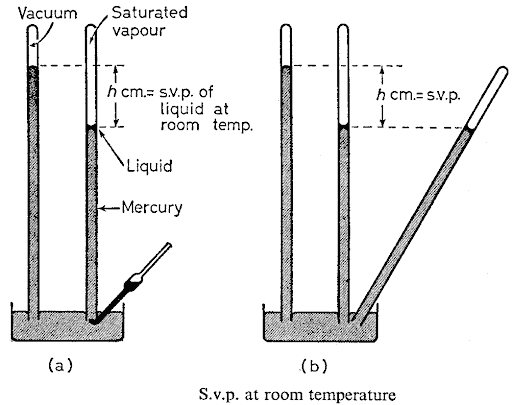
Now, we measure the saturation vapour pressure of a liquid
The vapour pressure of a liquid may be studied and measured by the aid of an ordinary simple barometer. The steps are so simple: 1- A very small quantity of hte chosen liquid is introduced into the lower end of the tube by means of a special bent pipette. In this way a small drop of liquid is allowed to rise up the mercury column.
2- On reaching the top it evaporates and the pressure exerted by the vapour depressed the column.
3- Now, if a sufficient liquid is added so that a small quantity remains on top of the mercury the space above will become saturated and the saturation vapour pressure may be measured by the total depression, h, of the column.
Tip: In order that this may be done accurately it is advisable to have a second barometer tube set up in the case the parometric pressure should alter during the course of the experiment. Also, the liquid used in the pipette should be freshly boiled so as to remove any dissolved air which would spoil the results.
Saturation vapour pressure does NOT depend on the volume
It is important to note that saturation vapour prssure at any given temperature is independent of the volume of the vapour so long as there is some free liquid present to ensure saturation condition.This may be demonstrated by tilting the barometer tube containing the vapour. The mercury rises up the tube but keeps at the same level. As the volume decreases, the excess vapour condenses back to liquid and the vapour pressure remains constant.
Variation of saturation pressure with temperature
The effect of temperature on saturated vapour pressure of a liquid may be studied by means of two barometer tubes placed side by side in a water bath. If water is the liquid to be investigated a little freshly boiled water is introduced into one of the tubes in the manner already described. The temperature of the water bath is then noted and the saturation vapour pressure found by measuring the difference, h in mm, in the mercury levels.
The water bath is stirred continuously and its temperature raised by passing in steam from a boiler. At 10 degree intervals readings of temperature and saturation vapour pressure are taken.
Variation of saturation vapour pressure with temperature:
From the results, a graph may be plotted, similar to that shown, although with this apparaus it is inadvisable to attempt to reach a temperature as high as 100 °C.
What happens when a liquid boils - ebullition-
If a liquid is heated its temperature begins to rise, and therefore the saturation vapour pressure will increase. Ultimately, the saturation vapour pressure becomes equal to the external atmospheric pressure. At this stage the further addition of heat will cause bubbles of vapour to form inside the body of the liquid and rise to the surface.This process is called boiling or ebullition.
The boiling point of a substance is defined as the temperature at which its saturation vapour pressure becomes equal to the external atmospheric pressure.
Variation of boiling point of water with pressure
Since the atmospheric pressure does not remain constant, the boiling point of water is liable to vary from day to day. Water boils at exactly 100 °C only when the barometric pressure is at the standard value of 760 mmHg. Tables are available which gives the boiling point at other pressures. These tables sho that the boiling point of water changes by approxiately 0.037 °C per mmHg change of pressure.Boiling under reduced pressure
Water can be made to poil without heating it simply by reducing the atmospheric pressure above it to a value less than the saturation vapour presure. This may be done with the aid of a filter pump. How? see below..
1- A flask containing some lukewarm water is fitted with a two-holed rubber bung through which passes a thermometer and a short glass tube.
2- When the flask is connected to a good filter pump the water begins to boil as soon as the pressure becomes less than the saturation vapour pressure corresponding to the temperature of water at the time.
Boiling under reduced pressure:
Since no heat is being supplied from outside, the necessary latent heat of vaporization has to come from the water itself. It therefore cools and the temperature indicated by the thermometer drops rapidly. To some extent this experiment illustrates how cooling occurs in the coils surrounding the freezing compartment of a refrigerator.