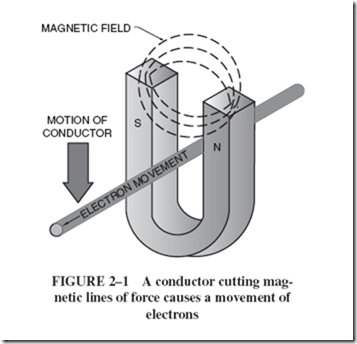
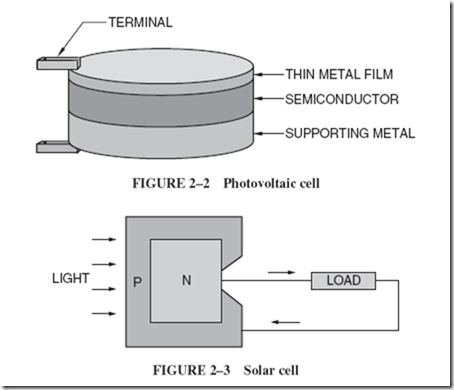
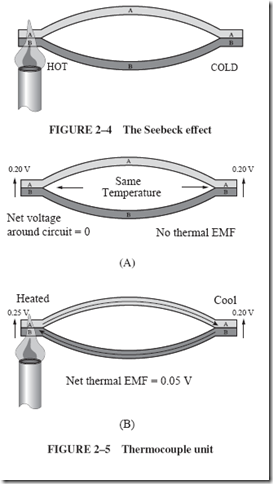
| The process begins with burning fossil fuels, either coal or gas, to create heat. The heat energy is used to produce steam for a turbine that, in turn, delivers mechanical energy to a generator. Part of this mechanical energy is converted into magnetism because a generator requires magnetic energy to produce the desired electrical energy. The flowchart that follows depicts this multiple conversion process. |