The definition of temperature
For several reasons, internal molecular energy which can be made available in the form of heat is the most important form of energy we have at our disposal ( see work, energy and power: pat 3 ) . Without the heat which comes to us from the sun, life on earth would soon becomes extinct.At one stage or another heat plays a part in the energy transformations by which we obtain electricity, which is the most useful and convenient of all forms of energy.
First, we need to know what the temperature is
The temprature of a substance is the number which expresses its degree of hotness on some chosen scale.Thus, the temperature of a bucketful of warm water is lower than that of a red-hot poker, but the water contains a much large quantity of internal energy than the poker.
Types of thermometer
Temperature is measured by means of a thermometer, of which a number of different types are available. Some depend on the expansion of a liquid when heated; others on the expansion of a compound strip of two metals. |
| Temperature scales |
 |
| Fahrenheit-to-Celsius |
Laboratory thermometers have cylindrical bulbs for easy insertion through holes in corks and have their scales engraved directly on the stem.
When the bulb is heated the liquid inside expands and the temperature is read at the level of the thread in the stem.
For some industrial purposes thermoelectric thermometers are used. These are based on the electric current which is generated when a junction of two metals is heated.
For accurate scientific work temperatures are often measured by a platinum wire contaied in a silica tube. Temperature changes may be calculated from the change in elecrical resistance of the platinum.
The fixed temperature points
The principle underlying the graduation of all types of thermometer is to choose two fixed and easily obtainable temperatures called the the upper and lower fixed pointsand then to divide the interval between them into a number of equal parts or degrees.
The upper fixed point is the temperature of steam from water boiling under standard atmospheric pressure of 760 mm Hg of mercury.
The temperature of the boiling water itself is not used as a fixed point for two reasons. First, local overheating may occur, accompained by "bumbing" as the water boils; secondly, any impurities which may be present will raise the boiling-boint.
The temperature of the steam just above the water will always be constant, and depends only on the barometric pressure at the time.
The lower fixed point is the temperature of pure melting ice.
The ice must be pure, since the presence of impureities will lower the melting-point.
Temperature scales
The difference in temperature between two fixed points is called the fundamental interval. This is divided into 100 equal degrees, the ice benig called 0 C and the steam point 100 C. This method of subdividing the interval was suggested by a Swedish astronomer named Celsius, and is now called the Celsius scale. Temperature on it are called " degrees Celsius" ( C).P.S. Actually the Celius scale as we know it today was first suggested by Linnaeus in 1745. Earlier, Celsius used similar scale in which the ice point was marked 100° and the stem point 0° .!
Another method of subdividing the interval was devised by Fahrenheit of Danzing. On the Fahrenheit scle the ice point is called 32 and the steam point 212, so there are 180 degrees in the fundamental interval.
These particular figures arose from the fact that Fahrenheit did not use the ice and steam fixed points, but chose instead the temperature of (a) an unspecified mixture of ice and salt, and (b) the human body, which he called 0 and 96 respectively. It so happened that, on his scale, pure ice melts at 32 F and water boils at 212 F.
The Fahrenheit scale is of historic importance only: it is no longer used in physics.
Six's maximum and minimum thermometer
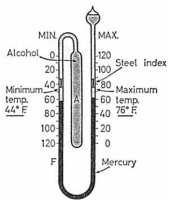 |
Six's maximum and minimum thermometer |
Invented by James Six towards the end of the eighteenth century, the thermometer consists of a fairly large cylindrical bulb A which originally contained alcohol, although oil of creosote is more generally used. This is connected by a U-shaped stem to a second bulb nearly full of the same liquid.
The bend of the U contains a thread of mercury. Two scales are provided, one against each limp of the tube so that the temperature may be read against either of the mercury levels. Resting on each of the mercury surfaces are small steel indexes provided with light springs to hold them in position in the stem.
Expansion or contraction of the liquid in A causes a movement of the mercury thread. Consequently, one or other index is pushed forward by the mercury and left in the extreme position reached. Thus, the lower end of the index on the left indicates the minimum and that on the right the maximum temperature attained.
After readings have been taken a small magnet is used to bring the indexes back into contact with mercury.