Measurements of gas pressure. Aneroid barometer and altimeter
A liquid finds it own level:
When water or any other liquid is poured into the communicating tubes it stands at the same level in each tube. This illustrates the popular saying that, water finds its own level.
When the liquid is at rest in the vessel the pressure must be the same at all points along the same horizontal level, otherwise the liquid would move until the pressure were equalized. The fact that the liquid stands at the same vertical height in all tubes whatever their shape confirms that, for a given liquid, the pressure at a point within it varies only with the vertical depth of the point below the surface of the liquid.
I appreciate your time. So if want to save this article as a pdf, feel free to download it: Measurements of gas pressure. Aneroid barometer and altimeter.
More articles:At this page- that is where you can find and download many articles : Articles in pdf
Measurement of gas pressure by the manometer
Torricelli's experiment. Simple barometer
Pascal's experiments with barometers
The aneroid barometer and altimeter
Measurement of gas pressure by the manometer
In the previous post ( Pressure in liquids and gases) it was stated that the atmospheric pressure was about 100 kilo newtons per m². Before explaining how this is measured let us make a short study of the manometer, an instrument for measuring the pressure of gas.
Manometer:
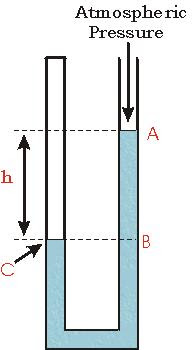
The manometer consists of a U-tube containing water. When both arms are open to the atmosphere the same atmospheric pressure is exerted on the water surfaces A and B, and these are at the same horizontal level.
In order to measure the pressure of the gas supply in the laboratory, the side A is connected to a gas-tap by a length of rubber tubing. When the tap is turned on the gas exerts pressure on the surface A, with the result that the level B rises until the pressure at C on the same horizontal level as A becomes equal to the gas pressure. Thus,
Pressure of gas = atmospheric pressure + pressure due to water column BC
It follows that the excess pressure, in N/m², of the gas above that of the atmosphere is given by the pressure of the water column BC, and is therefore equal to hρg as explained earlier.
The height, h, is called the head of water in the manometer and it is often convenient to express the excess pressure simply in terms of h only. In this case the units generally used are millimeters of water ( mm H2O ).
For measuring higher pressure than in the example above, mercury ( density 13.6 g /cm³) is used in the manometer, while for lower pressure a liquid such as xylene (density 0.88 g/cm³) is more suitable than water. We may therefore say,
excess pressure of gas supply = h ( in appropriate units).
Torricelli’s experiment. Simple barometer
About the middle of the seventeenth century an Italian scientist named Torricelli, living at Pisa, suggested an experiment to discount the theory that nature abhorred vacuum. Torricelli believed that nature’s supposed horror of a vacuum was caused simply by atmospheric pressure.
In a famous experiment, first performed in 1643, he set up the first barometer, an instrument for measuring the pressure of the air.
Torricelli's experiment:
How can you do this experiment:
1- In the laboratory a simple barometer can be made by taking a stout-walled glass tube about a meter long and closed at one end, and filling it almost to the top with clean mercury.
2-This is done with the aid of a small glass funnel and short length of rubber tubing.
3- Small air bubbles will generally be noticed clinging to the walls of the tube, and these must be removed.
4- With the finger placed securely over its open end, the tube is inverted several times so that the large air bubble left at the top of the tube travels up and down, collecting the small bubbles on its way.
5- More mercury is then added so that the tube is completely full.
6- The finger is again placed over the open end of the tube, while is now inverted and placed vertically with its end well below the surface of some mercury in a dish.
7-The finger is then removed and the column of mercury in the tube falls until the vertical difference in level between the surfaces of the mercury in tube and dish is about 760 mm.
The vertical height of the mercury column remains constant even when the tube is tilted, unless the top of the tube is less than 760 mm above the level in the dish, in which case the mercury completely fills the tube.
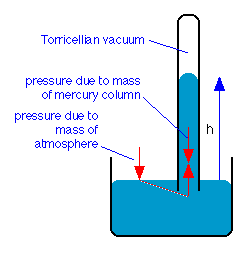
Torricelli explained that the column of mercury was supported in the tube by the atmospheric pressure acting on the surface of the mercury in the dish, and pointed out that small changes in the height of the column, which are noticed from day to day, are due to fluctuations in the atmospheric pressure.
The space above the mercury in the tube is called a Torricellian vacuum; it contains a little mercury vapor, and in this respect differs from a true vacuum.
Pascal’s experiments with barometers
Torricelli died a few years after the barometer experiment had been performed, and did not live to see his explanation of it, in terms of atmospheric pressure, generally accepted among scientists.
After Torricelli’s death Pascal repeated the experiment in France and set up two barometers.
The first of these was placed at the foot of a mountain in Auvergne called the Puy-de-Dome, while the other was carried up the mountainside and the height of the column read at intervals on the way up.
Owing to the decreasing height of the atmosphere above this barometer, its mercury column showed a progressive fall due to the reduced atmospheric pressure.
The barometer at the foot of the mountain showed practically no change.
It was this final experiment which brought about the downfall of the theory that nature abhors a vacuum and established the principle that the atmosphere exerts a pressure.
The aneroid barometer and altimeter
Barometers of the aneroid ( without liquids) type are commonly used as weather glasses, the idea being that low pressure, or a sudden fall in pressure, generally indicates unsettled weather while a rising barometer or high pressure is associated with fine weather.
The essential part of an aneroid barometer is a flat cylindrical metal box of capsule, corrugated for strength, and hermetically sealed after having been partially causes the box to cave in slightly, while a decrease allows it to expand.
The movements of the box are magnified by a system of levers and transmitted to a fine chain wrapped round the spindle of a pointer.
The chain is kept taut by means of a hairspring attached to the spindle, while the pointer moves over a suitably calibrated scale. Aneroid barometer movements are also used in the construction of altimeters for aircraft. In these the scale is calibrated in meters of ascent.
Roughly speaking the pressure falls by 10 mmHg per 120 m of ascent in the lower atmosphere.
The water barometer
Since the density of mercury is 13.6 g /cm³, it follows that if water was used as the liquid in a simple barometer the water column would have to be 76 × 13.6 cm = 10.3 m long!.
Such a barometer was constructed in the seventeenth century by von Guericke and fixed on the outside wall of his house. The upper level of the column was indicated by a small wooden float inside the tube on the surface of the water. With the aid of this barometer von Guericke made the first recorded scientific weather forecast. Having noted a sudden fall in the height of the water column, he correctly predicted the imminence of a severe storm.