Saturation vapour pressure illustration
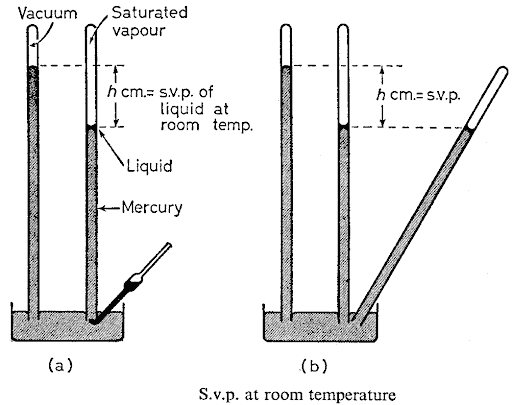
How liquids evaporate It is a matter of common knowledge that some water in an open vessel eventually dries up through evaporation. Liquids vary in the rate at which they evaporate at ordinary temperatures. They are said to be volatile. On the other hand, lubricating oil and mercury never seem to evaporate, however they are allowed to stand. When discussing the process of evaporating, we used the kinetic energy to explain how molecules escape from a liquid. If a liquid is heated, the energy which goes into it becomes mechanical energy in the molecules. More and more of the molecules gain enough kinetic energy to enable them to escape from the attraction of their neighbours and jump right out of the liquid. A rise in temperature is, therefore, accompained by an increase in the rate of evaporation. Saturated vapour pressure (s.v.p.) Let us suppose that some liquid is poured into a bottle which is then corked up. Owing to ...






